An investigation reveals the ability to reorder certain chemical elements that allows cheaper batteries, that last longer and support more recharge cycles.
Batteries are a fundamental element of mobile devices and vehicles. However, current technology has not yet managed to avoid its loss of efficiency and progressive deterioration. A work by the Japanese researcher Atsuo Yamada, from the University of Tokyo, with the collaboration of the Condensed Matter Chemistry Institute of Bordeaux and Marine Reynaud and Montse Casas-Cabanas, of the Spanish entity CIC energiGUNEa, has identified a capacity of certain elements that open the door to batteries with more capacity, more efficient and with more resistance to recharge cycles. It is what Casas-Cabanas describes as "atomic choreography".
"Energy is stored in the form of a chemical reaction that generates repulsions and destabilizes the elements. According to the material used in the battery, more or less changes are produced that degrade it ", explains the Spanish researcher. The work of the international team, published in Nature Communications, has identified a combination of materials that can be sorted during the loading process and that would allow the development of more sustainable and effective batteries.
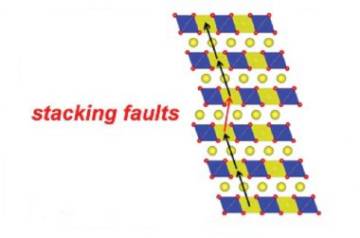
The material investigated is sodium oxide and ruthenium (Na 2 RuO 3 ), which has shown better reversibility. Its structure is laminar, meaning that the atoms are organized in different layers stacked on top of each other. "It's like a brick wall where the elements that make up the second row are slightly displaced to increase their resistance", simplifies Casas-Cabanas to explain how particles are sorted. This recomposition includes "planar defects", which is why it is not perfect. However, this feature, instead of being an inconvenience, has been revealed as an advantage to make the batteries more efficient.
"It is a new mechanism that implies an autoordination of the structure during the battery charging process, and that, in addition, is reversible, since when unloading it returns to the disordered structure of origin. This disorder is usually considered a defect, but it can be an opportunity ", affirms the researcher, who defines the process as" atomic choreography ".
The Basque research center (CIC energiGUNE) has developed the Faults computer program that has allowed us to analyze and understand this mechanism for the first time. The result is a more stable battery that avoids repulsions and degradation, a door to the development of sustainable batteries and greater capacity if research continues on chemical elements that include the same characteristics.
Challenges
"The challenge is that batteries with new materials work like lithium batteries, are cheaper and generate less impact. In addition, we can apply what we have already learned from existing ones, "says Casas-Cabanas.
Sodium batteries began to be investigated in the seventies of the last century, but the development of those composed of lithium relegated the advances. However, some companies have begun to develop batteries with this common element, abundant and cheap.
This is the case of the British Faradion , which markets sodium-based batteries for multiple devices, including vehicles, and which they claim are more durable, safer and cheaper than those commonly used. Or the French Tiamat , which advocates that its energy sources composed of sodium support faster recharges and a greater cycle of uses. Read about Facebook Scandal here.





COMMENTS